Bioprocess Technology Market Share, Size, Growth, Trends and Forecast 2025-2033
IMARC Group, a leading market research company, has recently releases a report titled “Bioprocess Technology Market Size, Share, Trends and Forecast by Product, Application, End User, and Region, 2025-2033.” The study provides a detailed analysis of the industry, including the global bioprocess technology market share, growth, size, trends and forecast. The report also includes competitor and regional analysis and highlights the latest advancements in the market.
Bioprocess Technology Market Highlights:
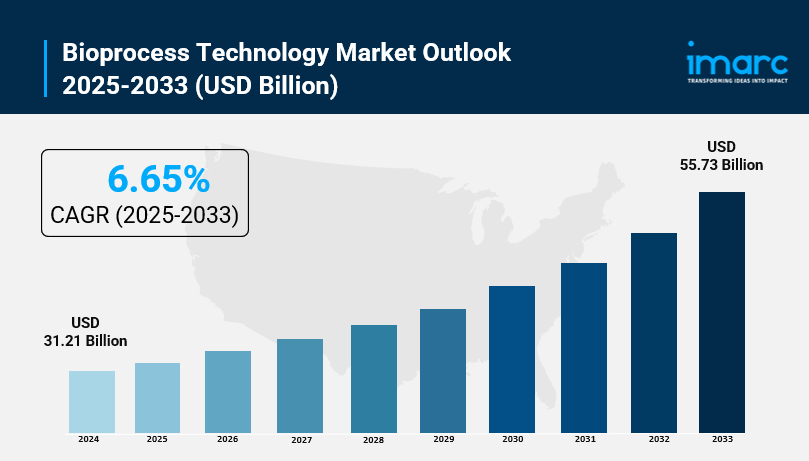
- Bioprocess Technology Market Size: Valued at USD 31.21 Billion in 2024.
- Bioprocess Technology Market Forecast: The market is expected to reach USD 55.73 billion by 2033, growing at an impressive rate of 6.65% annually.
- Market Growth: The bioprocess technology market is experiencing significant growth, driven by increased demand for biopharmaceuticals.
- Key Applications: Major applications include drug manufacturing, vaccine production, and the development of biologics.
- Technological Advancements: Innovations in bioprocessing techniques, such as single-use systems and automation, are enhancing efficiency and reducing costs.
- Regional Insights: North America holds a substantial market share, followed by Europe and Asia-Pacific, due to robust research and development activities.
- Market Challenges: Regulatory hurdles and high production costs pose challenges to market expansion.
- Future Trends: The market is expected to evolve with the integration of artificial intelligence and machine learning for process optimization.
Request for a sample copy of the report: https://www.imarcgroup.com/bioprocess-technology-market/requestsample
Our report includes:
- Market Dynamics
- Market Trends and Market Outlook
- Competitive Analysis
- Industry Segmentation
- Strategic Recommendations
Industry Trends and Drivers:
- The Shift to Advanced Biologics and Cell & Gene Therapies:
The bioprocess technology market is fundamentally driven by the revolutionary expansion of the biologics pipeline, moving beyond traditional vaccines and therapeutic proteins to complex molecules like monoclonal antibodies (mAbs), and emerging modalities such as cell and gene therapies (CGTs). These advanced therapies present unique manufacturing challenges, primarily due to their sensitivity, low expression titers, and stringent quality requirements. The shift mandates innovation in upstream processing, requiring highly specialized bioreactors and media for delicate cell lines. Downstream processing also requires bespoke solutions, moving away from high-volume, stainless-steel setups toward smaller-scale, closed, and automated systems suitable for personalized or niche-market drugs. This rapidly evolving and complex product landscape compels biopharmaceutical companies to adopt cutting-edge bioprocess technologies that can ensure scalability, purity, and regulatory compliance for these next-generation medicines. This investment ensures they can reliably bring these breakthrough treatments from lab bench to patient.
- Accelerated Adoption of Single-Use Bioprocessing Systems:
A major market trend is the rapid and widespread adoption of Single-Use Systems (SUS), or disposable technology, across both R&D and commercial manufacturing. SUS components, including bioreactors, mixers, filters, and fluid transfer lines, eliminate the need for costly and time-consuming sterilization (cleaning-in-place/sterilization-in-place, or CIP/SIP) required by traditional stainless-steel equipment. This significantly reduces downtime, lowers utility consumption, and, critically, minimizes the risk of cross-contamination between batches. For biopharmaceutical companies, this translates into faster facility construction, greater operational flexibility, and quicker changeovers, making SUS ideal for multi-product facilities, contract manufacturing organizations (CMOs), and the production of smaller-batch, high-value therapies like CGTs. While waste management remains a challenge, the compelling benefits in terms of efficiency, capital expenditure reduction, and speed-to-market continue to solidify SUS as a dominant driver of bioprocess technology investment, especially in emerging therapeutic fields.
- Integration of Automation and Continuous Bioprocessing:
The third key driver is the convergence of Industry 4.0 principles, primarily through the integration of advanced automation, digitalization, and continuous bioprocessing. Traditional batch processing is being challenged by continuous manufacturing (CM) methods, which run processes non-stop for weeks or months, leading to significantly higher yields, smaller footprint requirements, and superior product quality control. This CM approach is enabled by highly sophisticated sensors, Process Analytical Technology (PAT), and automated control systems that monitor and adjust parameters in real-time. Automation ensures consistency and reduces human error, while digital tools allow for comprehensive data analysis and predictive modeling. This shift is essential for achieving the efficiencies required to make biologics more affordable and accessible, and it compels bioprocess technology providers to deliver seamlessly integrated, digitally connected, and highly intelligent manufacturing platforms capable of meeting the stringent quality standards of regulators worldwide.
Bioprocess Technology Market Report Segmentation:
Breakup by Product:
- Instruments
- Bioprocess Analyzers
- Osmometers
- Bioreactors
- Incubators
- Others
- Consumables and Access
- Culture Media
- Reagents
- Others
Breakup by Application:
- Recombinant Proteins
- Monoclonal Antibodies
- Antibiotics
- Others
Breakup by End User:
- Biotechnology and Biopharmaceutical Companies
- Research and Academic Institutes
- Others
Breakup By Region:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
Who are the key players operating in the industry?
The report covers the major market players including:
- Advanced Instruments LLC
- Biopharma Dynamics Ltd.
- Danaher Corporation
- Hoffmann-La Roche AG
- Lonza Group AG
- Sartorius AG
- Thermo Fisher Scientific Inc.
- Univercells Technologies
Ask Analyst For Request Customization: https://www.imarcgroup.com/request?type=report&id=12992&flag=E
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provides a comprehensive suite of market entry and expansion services.
IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact US:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-201971-6302
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Jogos
- Gardening
- Health
- Início
- Literature
- Music
- Networking
- Outro
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


