Biosimilar Market is Projected to Reach USD 185.1 Billion by 2033 | At CAGR 24.1%
Overview of the Biosimilar Market:
The biosimilar market refers to biologic medical products that are highly similar to already approved reference biologics in terms of safety, purity, and efficacy. Biosimilars are playing a crucial role in expanding access to advanced therapies while reducing healthcare costs. They are widely used in treating chronic diseases such as cancer, autoimmune disorders, diabetes, and rheumatoid arthritis. The market is experiencing strong growth, driven by patent expirations of blockbuster biologics, rising healthcare expenditures, and the increasing demand for affordable treatment options. Regulatory approvals and supportive government initiatives across major regions are further accelerating adoption.
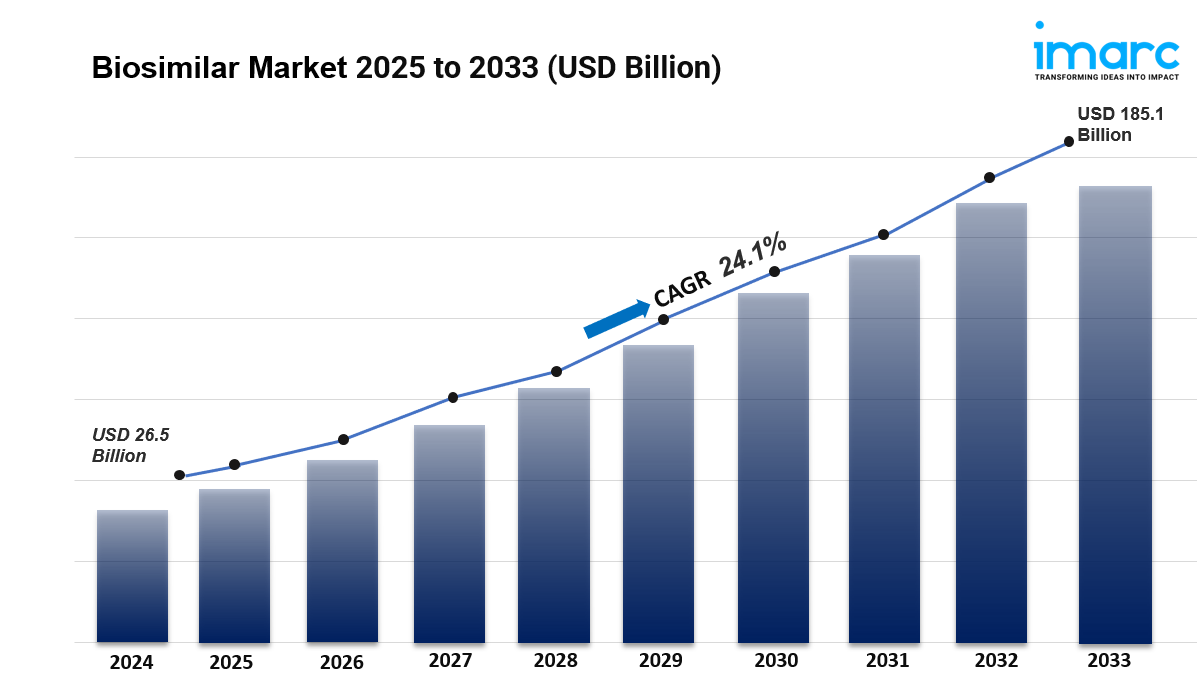
The global biosimilar market size was valued at USD 26.5 Billion in 2024. Looking forward, IMARC Group estimates the market to reach USD 185.1 Billion by 2033, exhibiting a CAGR of 24.1% from 2025-2033. Europe currently dominates the market. The expiration of patents for major biological drugs, growing awareness about the efficacy and cost-effectiveness of biosimilars, the rising prevalence of chronic diseases worldwide, and continual advancements in biopharmaceutical manufacturing technologies are some of the major factors boosting the biosimilar market share.
Key Trends:
-
Patent Expirations of Biologics: Expiry of patents for major biologic drugs is opening opportunities for biosimilar manufacturers.
-
Regulatory Harmonization: Streamlined approval processes by the FDA, EMA, and other authorities are fostering quicker market entry.
-
Price Competition & Cost Savings: Growing acceptance of biosimilars as cost-effective alternatives is boosting adoption among healthcare providers.
-
Strategic Collaborations: Partnerships, licensing agreements, and joint ventures between pharma companies are expanding global reach.
-
Rising Adoption in Oncology & Autoimmune Therapies: Oncology, rheumatology, and diabetes segments remain at the forefront of biosimilar demand.
Growth Drivers:
-
Rising Prevalence of Chronic Diseases: Growing cases of cancer, diabetes, and autoimmune disorders are increasing the need for affordable biologic therapies.
-
Healthcare Cost Containment: Governments and insurers are promoting biosimilars to reduce the financial burden of biologic treatments.
-
Expanding Biopharmaceutical R&D: Technological advancements in biologics manufacturing are enabling efficient production of high-quality biosimilars.
-
Increasing Physician & Patient Acceptance: Greater awareness and proven clinical efficacy are driving confidence in biosimilars.
-
Emerging Markets Growth: Rising healthcare investments in Asia-Pacific, Latin America, and the Middle East are creating new opportunities.
Factors Affecting the Growth of the Biosimilar Market Industry:
Increasing Acceptance of Biosimilars:
The biosimilar market is expanding rapidly as healthcare providers, payers, and patients increasingly recognize their value. Growing awareness of biosimilars as cost-effective alternatives to expensive biologics is driving adoption across the healthcare landscape. Strong clinical evidence confirms their safety and efficacy, helping address concerns about switching from reference biologics. Regulatory bodies such as the FDA and EMA have established clear approval frameworks, further strengthening confidence in biosimilar quality and reliability. With rising global drug costs, biosimilars are emerging as an essential solution to improve access to life-saving therapies—particularly for chronic conditions such as cancer, autoimmune diseases, and diabetes. As physicians become more willing to prescribe biosimilars and positive patient experiences accumulate, acceptance is expected to accelerate, fueling long-term market growth.
Expanding Therapeutic Applications:
The scope of biosimilar applications is broadening beyond oncology and inflammatory diseases into areas such as metabolic disorders, ophthalmology, and rare diseases. This expansion is largely driven by the increasing expiration of biologic patents, which is opening opportunities for biosimilar manufacturers to enter new therapeutic markets. Advances in biomanufacturing technologies are also enabling the production of high-quality biosimilars capable of competing effectively with original biologics. With more biosimilars gaining regulatory approval, healthcare providers now have access to a wider range of treatment options, ultimately improving patient outcomes. As adoption grows across new therapeutic areas, biosimilars are expected to intensify competition, reduce treatment costs, and enhance accessibility to vital therapies, further propelling market growth.
Strategic Collaborations and Partnerships:
Collaborations among pharmaceutical companies, biotechnology firms, and research institutions are playing a pivotal role in shaping the biosimilar market. These partnerships allow stakeholders to pool expertise, share resources, and leverage advanced technologies, thereby reducing R&D expenses and accelerating product development. Large pharmaceutical companies often collaborate with smaller, specialized biotech firms to gain access to innovative platforms and speed up commercialization efforts. In addition, such alliances provide broader distribution networks and deeper market insights, enabling companies to better position their products in an increasingly competitive landscape. As the biosimilar industry evolves, strategic collaborations will remain essential to driving innovation, improving efficiency, and building a robust ecosystem for the development and adoption of biosimilars.
Request to Get the Sample Report: https://www.imarcgroup.com/biosimilar-market/requestsample
Biosimilar Market Report Segmentation:
Breakup By Molecule:
- Infliximab
- Insulin Glargine
- Epoetin Alfa
- Etanercept
- Filgrastim
- Somatropin
- Rituximab
- Follitropin Alfa
- Adalimumab
- Pegfilgrastim
- Trastuzumab
- Bevacizumab
- Others
Infliximab accounts for the majority of shares due to its widespread use in treating chronic autoimmune diseases such as rheumatoid arthritis and Crohn's disease.
Breakup By Indication:
- Auto-Immune Diseases
- Blood Disorder
- Diabetes
- Oncology
- Growth Deficiency
- Female Infertility
- Others
Autoimmune diseases dominate the market as biologics, and biosimilars are highly effective in managing conditions like rheumatoid arthritis, psoriasis, and inflammatory bowel disease.
Breakup By Manufacturing Type:
- In-house Manufacturing
- Contract Manufacturing
In-house manufacturing represents the majority of shares because it enables better control over production quality and reduces reliance on third-party manufacturers, ensuring compliance with strict regulatory standards.
Breakup By Region:
- Europe
- Germany
- France
- Italy
- Spain
- United Kingdom
- Rest of Europe
- United States
- Japan
- India
- South Korea
- Rest of the World
Europe holds the leading position due to its well-established regulatory pathways for biosimilars and strong government support for biosimilar adoption.
Top Biosimilar Market Leaders:
The biosimilar market research report outlines a detailed analysis of the competitive landscape, offering in-depth profiles of major companies.
Some of the key players in the market are:
- Sandoz International GmbH
- Pfizer Inc.
- Teva Pharmaceutical Industries Limited
- Celltrion Inc.
- Biocon Limited
- Samsung Biologics
- Amgen, Inc.
- Dr. Reddy's Laboratories Limited
- Stada Arzneimittel Ag.
Speak to An Analyst: https://www.imarcgroup.com/request?type=report&id=497&flag=C
If you need specific information that is not currently within the scope of the report, we will provide it to you as a part of the customization.
About Us
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services.
IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact US:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-201971-6302
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Giochi
- Gardening
- Health
- Home
- Literature
- Musica
- Networking
- Altre informazioni
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


