Huge Revenue Jump Predicted in Global In-Vitro Diagnostic Market Between 2020 and 2030
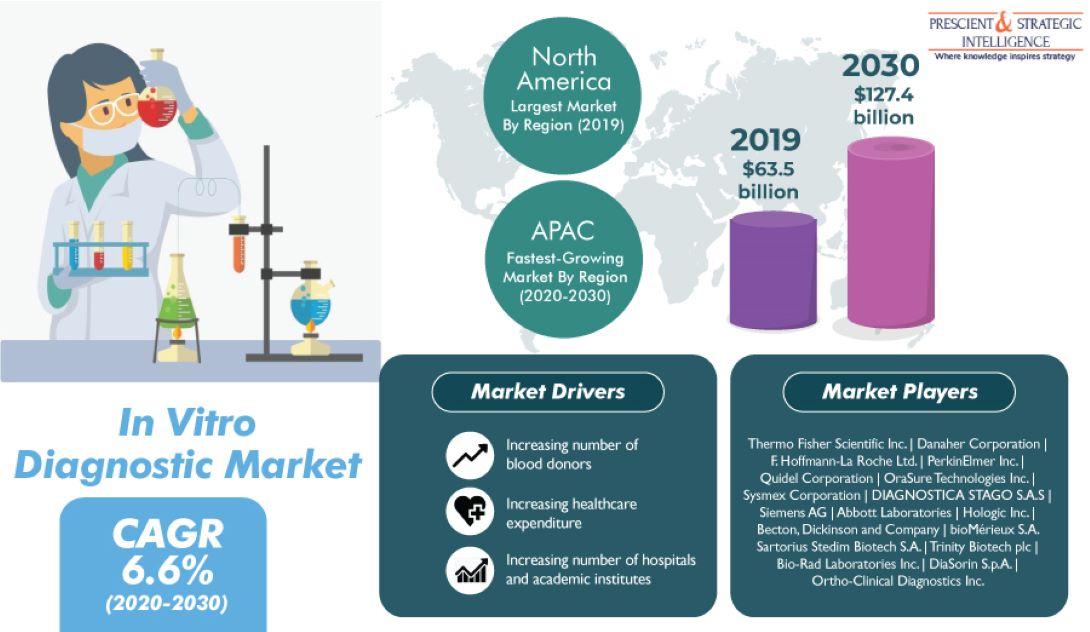
The global in-vitro diagnostic (IVD) market reached a valuation of $63.5 billion in 2019 and is predicted to progress at a CAGR of 6.6% between 2020 and 2030. According to the estimates of P&S Intelligence, a market research firm based in India, the market would generate a revenue of $127.4 billion by 2030. The key factor driving the progress of the market is the rising enactment of strict regulations and policies in several countries regarding blood sample contamination.
Besides the aforementioned factor, the growing incidence of chronic diseases, the surging number of blood donors, rapid advancements and innovations in the IVD technology, the increasing number of academic institutions and hospitals, and soaring healthcare expenditure in various countries are also fueling the expansion of the in-vitro diagnostic market across the world. As per the International Diabetes Federation (IDF) Atlas 9th edition, 352 million people in the age group 20—64 were diagnosed with diabetes in 2019.
Furthermore, this number is predicted to grow to 486 million by 2045. As per the findings of the World Health Organization (WHO), cancer claimed as many as 9.6 million lives in 2018. The Joint United Nations Programme on HIV/AIDS (UNAIDS) found that 37.9 million people around the world suffered from HIV/AIDS in 2018. The high occurrence of these diseases is boosting the demand for IVD testing, which is, in turn, propelling the growth of the market.
To download a free sample copy of this report@ https://www.psmarketresearch.com/market-analysis/in-vitro-diagnostic-market/report-sample
Furthermore, the increasing number of blood donors in several countries is bolstering the market growth. As per the WHO, 118.4 million units of blood were donated throughout the world in 2018. According to various reports, there were nearly 13,300 blood centers in 169 countries and approximately 106 million blood donations were recorded in the same year. In addition to this, over 90% of the blood supply in as many as 79 countries was from voluntary unpaid blood donations.
Based on offering, the in-vitro diagnostic market is divided into software & services, instruments, and reagents & kits. Out of these, the reagents & kits category is predicted to dominate the market in the future years. The increasing number of IVD tests being performed all over the world, because of the ballooning prevalence of infectious and chronic diseases and the surging number of research activities, is driving the advancement of this category in the market in the forthcoming years.
Geographically, the in-vitro diagnostic market is predicted to exhibit the fastest growth in the Asia-Pacific (APAC) region in the upcoming years. This would be because of the existence of leading market players in the region and their focus on various strategic development activities in order to improve their market position. For example, according to Abbott Laboratories, in October 2019, the Australian Red Cross Blood Service signed a multi-year agreement for Alinity system, the organization’s plasma and blood screening technology.
Hence, it can be said without any hesitation that the market would boom in the future years, primarily because of the increasing requirement for blood donations and the growing enactment of several strict policies regarding blood contamination in several countries around the world.
SOURCE: P&S Intelligence

- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Jeux
- Gardening
- Health
- Domicile
- Literature
- Music
- Networking
- Autre
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


