South Korea Biosimilar Market Size, Share, Latest Insights and Forecast 2025-2033
IMARC Group has recently released a new research study titled “South Korea Biosimilar Market Size, Share, Trends and Forecast by Molecule, Indication, Manufacturing Type, and Region, 2025-2033”, offers a detailed analysis of the market drivers, segmentation, growth opportunities, trends and competitive landscape to understand the current and future market scenarios.
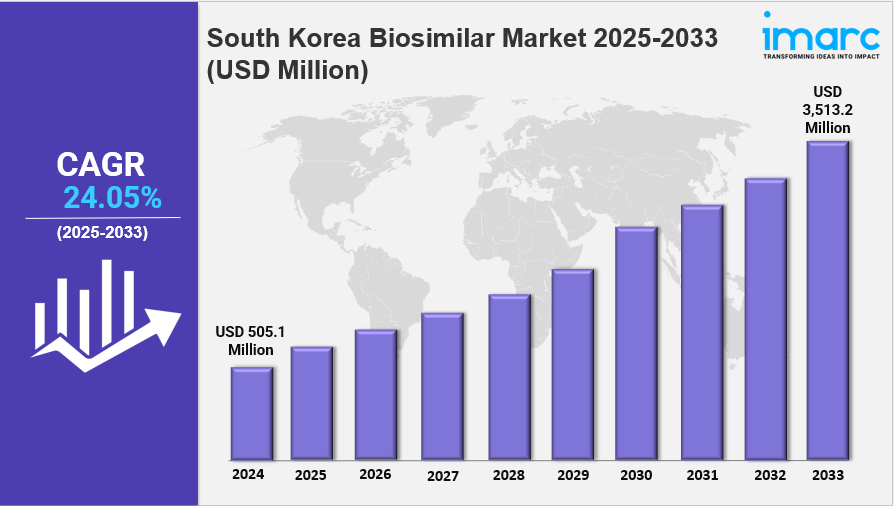
South Korea Biosimilar Market Overview
The South Korea biosimilar market size was valued at USD 505.1 Million in 2024. It is forecasted to expand to USD 3,513.2 Million by 2033, growing at a CAGR of 24.05% from 2025 to 2033. This growth is driven by favourable government policies, domestic R&D, high-quality manufacturing capacity, and increasing acceptance of biosimilars among healthcare stakeholders.
Study Assumption Years
- Base Year: 2024
- Historical Years: 2019-2024
- Forecast Period: 2025-2033
South Korea Biosimilar Market Key Takeaways
- Current Market Size: USD 505.1 Million in 2024
- CAGR: 24.05% during 2025-2033
- Forecast Period: 2025-2033
- The South Korean government supports biosimilars through structured policies, reimbursement mechanisms, and regulatory pathways for faster market access.
- Local R&D and manufacturing capacity have been bolstered, with firms deploying advanced bioprocessing technologies and global GMP compliance.
- Domestic companies, universities, and public-private partnerships actively collaborate to innovate biosimilar drugs across multiple therapeutic classes.
- Recent approvals of biosimilars like Celltrion’s denosumab versions and Dong-A ST’s ustekinumab biosimilar exemplify the growing pipeline.
- Strategic collaborations, such as between Samsung Biologics and Pfizer, enhance large-scale commercial manufacturing capacity.
Sample Request Link: https://www.imarcgroup.com/south-korea-biosimilar-market/requestsample
Market Growth Factors
The South Korea biosimilar market is primarily driven by government initiatives and policy support. The government has established a well-structured framework aimed at enhancing healthcare affordability and sustainability by promoting biosimilars. Incentive programs motivate local manufacturers to develop biosimilars of costly biologics nearing patent expiration. Streamlined regulatory pathways facilitate faster, cost-effective market entry through reference product comparability and bridging clinical studies. Public hospitals and insurance schemes incorporate biosimilars in formularies via reimbursement mechanisms favoring biosimilar pricing tiers. The Ministry of Food and Drug Safety’s clear guidelines for quality, safety, and interchangeability build clinician confidence and enable market penetration through substitution protocols and hospital incentives. This comprehensive policy ecosystem significantly propels market growth by easing producer viability and patient access.
Another key growth driver is the substantial investment South Korea has made in expanding local R&D and domestic manufacturing capacity. Companies and universities actively collaborate on biosimilar development across oncology, autoimmune, and endocrine therapeutic classes by leveraging advanced bioprocessing platforms and technology transfers. Production facilities comply with global GMP standards, supporting both domestic use and exports. Local firms scale capacity using single-use bioreactors, intensified fermentation, and automation, achieving cost efficiencies and production consistency. The ecosystem also includes CDMOs, CROs, and biosimilarity testing organizations, enabling rapid development cycles. Global partnerships further strengthen co-development and promotion of biosimilars abroad. The launch of FDA-approved biosimilars such as Dong-A ST’s ustekinumab biosimilar highlights growing homegrown innovation and reduced reliance on foreign licensing.
The increasing acceptance of biosimilars among clinicians, payers, and patients further boosts the market. Awareness and confidence in biosimilars have grown due to proven efficacy, safety, and cost benefits, supported by government policies and clear regulatory standards. This acceptance drives greater market penetration, reimbursement inclusion, and usage in clinical practice. The first approvals of biosimilars for conditions like osteoporosis, cancer-related bone metastasis, and autoimmune diseases signify expanding therapeutic options. Improved manufacturing readiness and integrated R&D pipelines ensure continuous supply and product availability, fostering competitive pricing and broader adoption. Such factors reinforce biosimilar market growth prospects in South Korea through the forecast period.
Market Segmentation
Molecule Insights:
- Infliximab: Biosimilars related to this monoclonal antibody.
- Insulin Glargine: Biosimilar forms for diabetes treatment.
- Epoetin Alfa: Biosimilars for anemia management.
- Etanercept: Biosimilars for autoimmune disease indications.
- Filgrastim: Biosimilars supporting neutropenia treatment.
- Somatropin: Biosimilars for growth deficiency disorders.
- Rituximab: Biosimilars targeting certain cancers and autoimmune conditions.
- Follitropin Alfa: Biosimilars used in female infertility treatments.
- Adalimumab: Biosimilars for autoimmune diseases.
- Pegfilgrastim: Biosimilars for neutropenia.
- Trastuzumab: Biosimilars for oncology indications.
- Bevacizumab: Oncology biosimilars.
- Others: Other biosimilar molecules covered.
Indication Insights:
- Auto-Immune Diseases: Biosimilars for managing autoimmune conditions.
- Blood Disorder: Biosimilars for hematologic conditions.
- Diabetes: Biosimilars relating to diabetic treatment.
- Oncology: Cancer-related biosimilars.
- Growth Deficiency: Biosimilars supporting growth hormone deficiencies.
- Female Infertility: Biosimilars used in fertility treatments.
- Others: Additional indications covered.
Manufacturing Type Insights:
- In-house Manufacturing: Biosimilars produced internally by companies.
- Contract Manufacturing: Biosimilars produced by third-party manufacturers.
Ask For Analyst- https://www.imarcgroup.com/request?type=report&id=39266&flag=C
Regional Insights
The key regions covered in the South Korea biosimilar market include Seoul Capital Area, Yeongnam (Southeastern Region), Honam (Southwestern Region), Hoseo (Central Region), and others. Overall, the South Korean biosimilar market benefits from well-established industrial clusters and infrastructure particularly in major regions like the Seoul Capital Area, supporting growth through domestic manufacturing capacity and healthcare demand.
Recent Developments & News
On June 8, 2023, Samsung Biologics and Pfizer announced a strategic collaboration for long-term commercial manufacturing of Pfizer’s multi-product biosimilars portfolio, including oncology, inflammation, and immunology treatments. Samsung Biologics plans to use its newly completed Plant 4 in Incheon for large-scale manufacturing. This partnership aims to improve global access to medicines and address emerging health challenges through joint efforts.
Key Players
- Celltrion
- Dong-A ST
- Samsung Biologics
- Pfizer
About Us
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us
IMARC Group,
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No: (D) +91 120 433 0800
United States: +1-201971-6302
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Παιχνίδια
- Gardening
- Health
- Κεντρική Σελίδα
- Literature
- Music
- Networking
- άλλο
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness



