IPV Vaccines Market - Global Industry Growth & Trend Analysis
Introspective Market Research, a global authority on public health and pharmaceutical market dynamics, today announced the publication of its definitive report on the Global Inactivated Poliovirus (IPV) Vaccines Market. This critical sector is fundamental to the Global Polio Eradication Initiative (GPEI), covering the supply and distribution of injectable vaccines used to provide immunity against poliomyelitis, specifically designed for safety across all populations, including the immunocompromised.
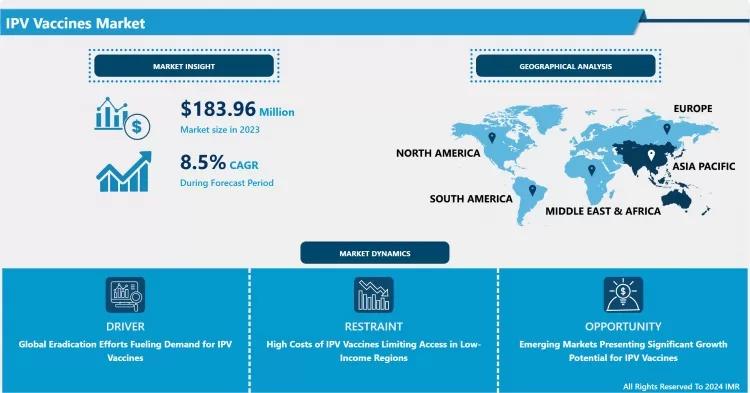
The Global IPV Vaccines Market size was valued at USD 183.96 Million in 2023 and is strategically projected to reach USD 383.35 Million by 2032. This robust expansion is forecasted to progress at a Compound Annual Growth Rate (CAGR) of 8.50% over the analysis period from 2024 to 2032. The single most important factor driving this growth is the Global Eradication Efforts Fueling Demand for IPV Vaccines, particularly as countries phase out the use of the oral poliovirus vaccine (OPV) due to the risk of vaccine-derived poliovirus.
Quick Insights: Global IPV Vaccines Market (2024–2032)
|
Metric |
Insight |
|
2023 Market Valuation |
USD 183.96 Million |
|
Projected 2032 Valuation |
USD 383.35 Million |
|
CAGR (2024-2032) |
8.50% |
|
Dominant Vaccine Type |
Conventional IPV (Widespread government contract use) |
|
Leading Age Group |
Infants (Core immunization target) |
|
Largest Regional Market |
North America (Highest per-dose pricing and established universal programs) |
|
Key Market Driver |
Polio Eradication Programs and the OPV-to-IPV Switch |
|
Key Opportunity |
Integration into Combination Vaccines |
Segmentation Deep Dive: Infants and Public Health Agencies Drive Volume
The market’s structure is intrinsically linked to mandatory global immunization policies:
· By Age Group: The Infants segment currently holds the largest market share. IPV is administered in multiple doses as part of routine childhood immunization schedules globally, making newborns and infants the primary consumer group. The consistent global birth rate ensures continuous baseline demand for this segment.
· By Vaccine Type: Conventional IPV still dominates due to massive procurement contracts by global organizations for use in national immunization programs. However, Enhanced IPV formulations are gaining traction for their potential to offer better immunogenicity or require fewer doses, optimizing supply chains, especially in resource-limited settings.
· By End User: Public Health Agencies and Vaccination Centers are the most dominant end-users. Unlike many other pharmaceuticals, the IPV market is overwhelmingly government-driven, relying on large-scale tenders from organizations like the WHO, UNICEF, and national health ministries for mass distribution.
How is Combination Vaccine Integration Streamlining Global Immunization Schedules?
A key trend fundamentally reshaping the market is the Increasing integration of IPV into combination vaccines. Combination vaccines—such as DTaP-IPV-Hib (Diphtheria, Tetanus, Pertussis, IPV, and Haemophilus influenzae type b)—offer several critical advantages:
1. Improved Compliance: Consolidating multiple injections into one shot drastically reduces the number of clinic visits required, significantly boosting compliance rates, especially in regions with fragmented healthcare access.
2. Reduced Supply Chain Complexity: Fewer individual vials need to be managed, simplifying procurement and reducing the strain on the Cold Chain Infrastructure which is vital for maintaining vaccine viability during transport and storage.
The development of new multi-component combinations that include IPV is a major focus for pharmaceutical innovation, aiming to make global immunization efforts more efficient and less burdensome on public health systems.
Expert Insight: The Delicate Balance of Safety and Accessibility
“The market is currently navigating a critical period: the final stages of polio eradication mean shifting the world away from OPV and toward the superior safety profile of IPV, which cannot cause vaccine-derived poliovirus,” says Dr. Aris Thorne, Principal Consultant at Precedence Research. “The challenge is that IPV is significantly more expensive and often requires a robust cold chain, which limits access in the very countries that need it most to halt residual poliovirus outbreaks. Manufacturers who can innovate toward thermostable IPV formulations or drastically reduce production costs—allowing for equitable pricing under GPEI agreements—will define the success of this market over the next decade.”
Regional Analysis: North America’s Value vs. Asia Pacific’s Volume Potential
North America holds the largest market share in terms of value, primarily due to its advanced immunization programs, mandatory IPV schedules, and the ability of manufacturers to command high prices in the US market. The region’s sophisticated regulatory environment also drives early adoption of Enhanced IPV formulations.
However, the Asia-Pacific (APAC) region is expected to demonstrate substantial future volume growth. This is driven by:
1. Massive Scale of Immunization: Countries like India and China have immense birth cohorts requiring mandatory IPV doses.
2. Government Focus: Aggressive government programs aimed at improving childhood health and immunization coverage.
3. Local Manufacturing: Companies like the Serum Institute of India (a major global supplier) are strategically positioned to meet the high-volume demand and offer cost-effective options required by public health contracts.
Major Breakthroughs and Corporate Strategy
Key market players are focusing heavily on next-generation IPV and combination products:
· Sanofi (France): A dominant player globally, offering both stand-alone IPV and robust combination vaccines, and investing heavily in optimizing manufacturing processes to meet GPEI supply quotas efficiently.
· GlaxoSmithKline plc (GSK): Continues to strengthen its pediatric vaccine portfolio with integrated combination products that include IPV, targeting both mandatory and private markets.
· Serum Institute of India Pvt. Ltd. (SII): This company is vital for global supply, focusing on high-volume, cost-effective production to support low- and middle-income countries, which is essential for the long-term success of the eradication strategy.
Challenges and Cost Pressures
The most significant market restraint is the High Costs of IPV Vaccines Limiting Access in Low-Income Regions. IPV is fundamentally more complex and expensive to produce than OPV. This cost difference necessitates continuous funding from international organizations (like Gavi, the Vaccine Alliance) to subsidize procurement. Furthermore, any disruption to the Cold Chain (the temperature-controlled supply chain) in remote or poorly infrastructured areas poses a constant threat to vaccine efficacy and distribution success.
Case Study: The Final IPV Rollout in Sub-Saharan Africa
Following a WHO recommendation, a consortium of GPEI partners began a large-scale transition from OPV to a sequential IPV regimen in a key Sub-Saharan African country with historic polio endemicity. The initial six-month phase faced challenges due to the higher cost and limited storage capacity for the IPV. The solution involved:
1. Pre-Qualified Multi-Dose Vials: Using multi-dose vials to reduce waste and optimize storage space.
2. Solar-Powered Cold Boxes: Deploying solar-powered refrigeration units to maintain the required cold chain temperature in rural clinics.
This strategic deployment resulted in successful administration to over 85% of the target infant population, demonstrating that innovative logistical solutions can effectively mitigate the cost and infrastructure challenges of IPV in high-risk regions.
Call to Action
Stay Ahead of the Curve: Download the Full IPV Vaccines Market Report Access critical data on market volumetric analysis, regional procurement trends, competitive benchmarking of major manufacturers (including Sanofi, GSK, and SII), and strategic insights into the transition phase of the Global Polio Eradication Initiative.
[Click Here to Request Sample Report] (Simulated Link: /reports/ipv-vaccines-market/request-sample)
About Introspective Market Research
Introspective Market Research (IMR) is a trusted, data-driven provider of comprehensive market intelligence, specializing in sectors critical to global health and infrastructure, including Pharmaceuticals, Biotechnology, and Public Health. Our reports empower governmental and corporate clients with the precise data needed for strategic planning and informed decision-making.
Contact: Introspective Market Research Phone: +91-74101-03736
Email: sales@introspectivemarketresearch.com
Website: https://introspectivemarketresearch.com/
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Spellen
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


