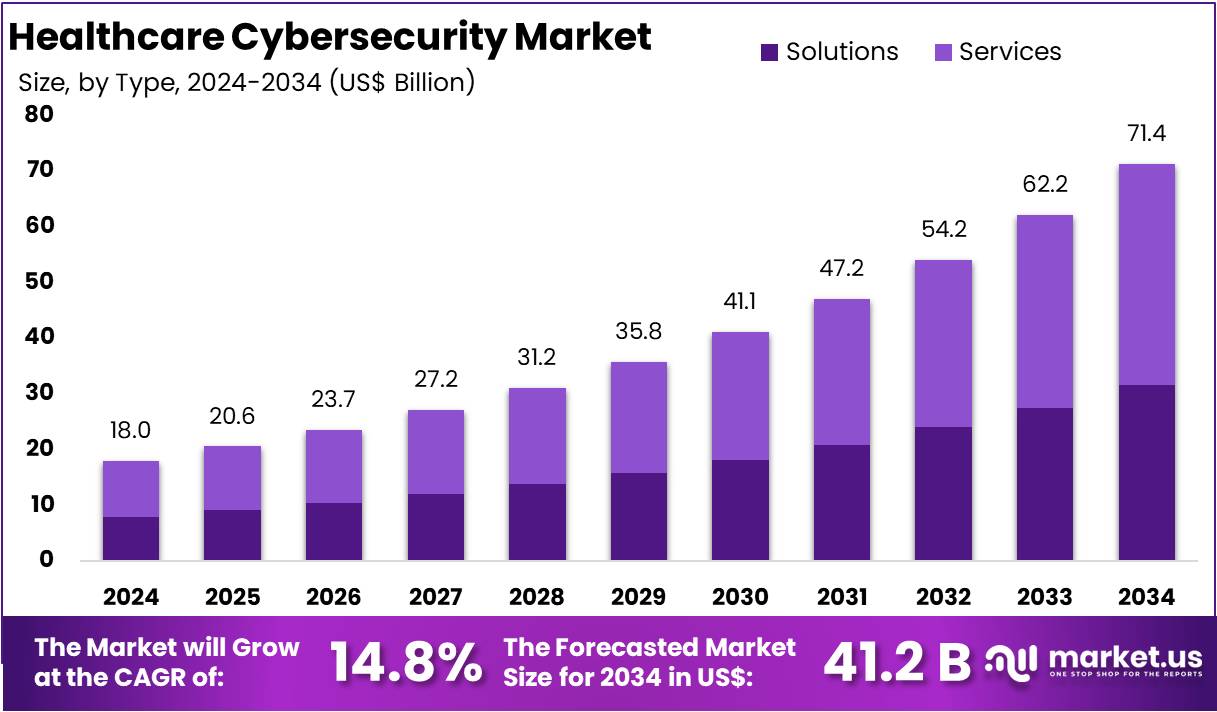
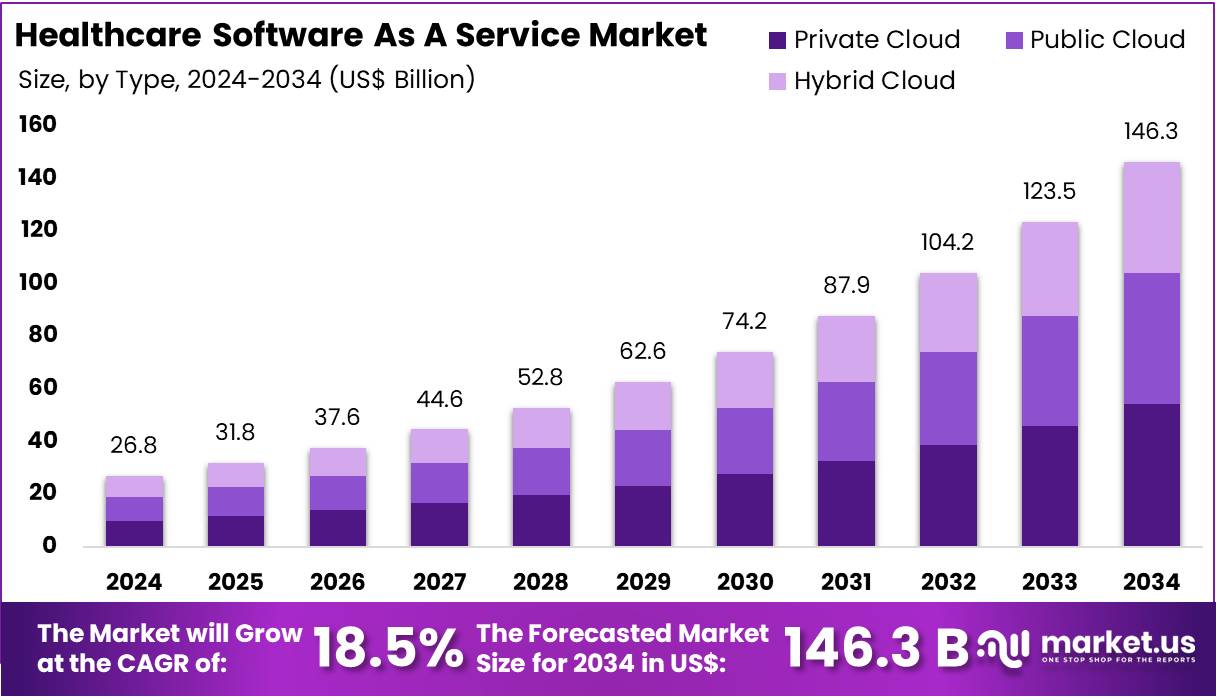
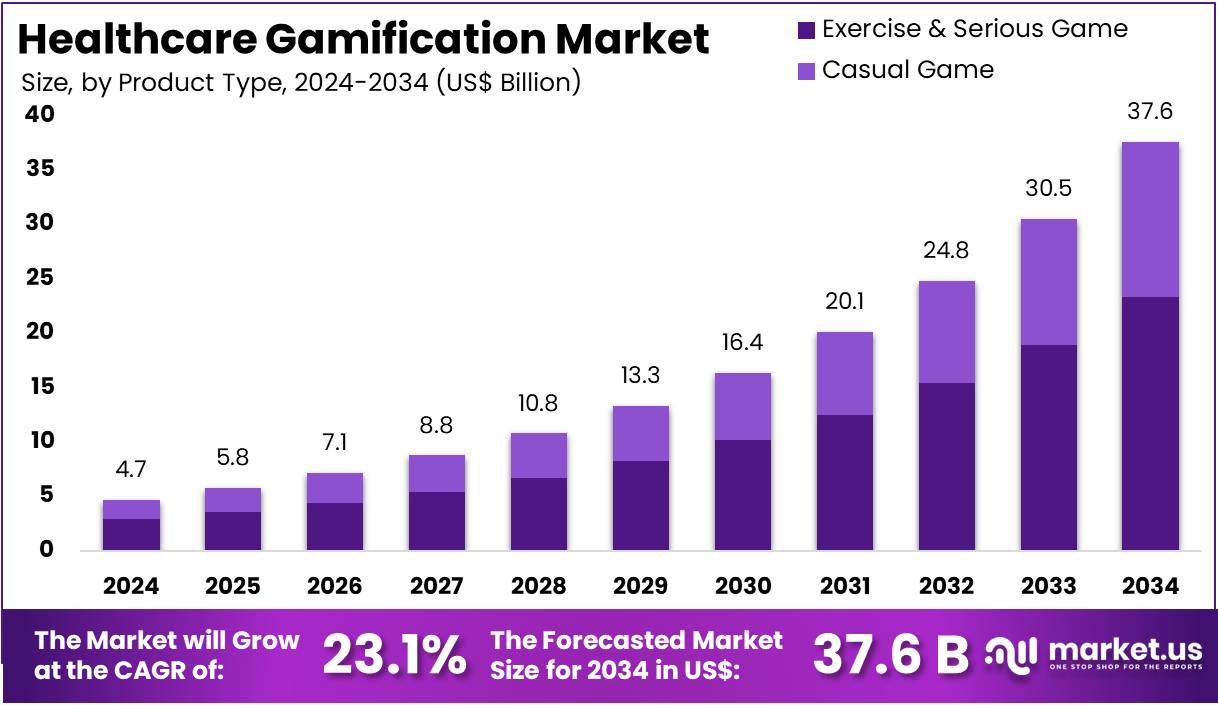
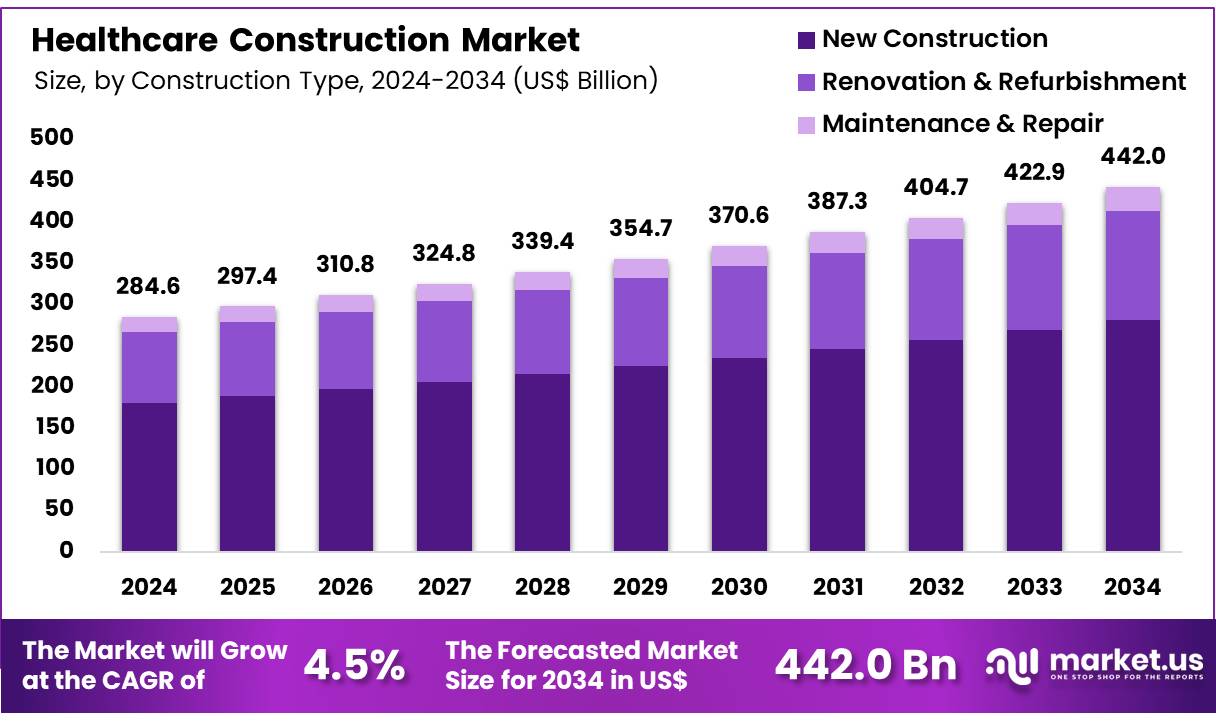
The Glucose Sensor Market is on track for substantial expansion as global healthcare systems intensify their focus on diabetes management and continuous monitoring. Valued at USD 9.7 billion in 2025, the market is projected to reach USD 22.2 billion by 2035, reflecting a steady 8.5% CAGR. With non-invasive sensors leading product adoption and demand accelerating across North America, Asia-Pacific, and Europe, growth prospects remain firmly positive.
Explore trends before investing – request a sample report today!
https://www.futuremarketinsights.com/reports/sample/rep-gb-1183
Rising Disease Burden and Real-Time Monitoring Fuel Market Expansion
Increasing global diabetes prevalence, paired with the shifting emphasis toward proactive care, is significantly driving market penetration. Hospitals, clinics, and homecare users increasingly recognize the value of continuous, real-time glucose readings in reducing complications, improving glycemic control, and supporting long-term health outcomes. Parallel to this, technological advancements—such as microfluidic sensing, optical technologies, and seamless wearable integrations—are boosting accuracy and consumer acceptance.
As government health bodies reinforce reimbursement policies supporting remote and continuous monitoring, adoption rates continue to climb. This convergence of medical need, policy direction, and emerging technology forms a powerful catalyst for sustained market growth.
Non-Invasive Innovations Unlock a New Era in Monitoring
Non-invasive glucose monitoring is emerging as the most influential trend shaping the industry. This category is projected to capture 57.9% of total market share in 2025, driven by the rising consumer preference for painless, easy-to-use solutions. New-generation sensors utilizing optical and transdermal technologies are entering the mainstream, delivering measurements without finger pricks or implanted components.
The appeal of these devices extends beyond comfort—they offer continuous data streams, reduce infection risks, and increase adherence rates. Wearable platforms launched in 2025, offering rechargeable systems and mobile app connectivity, are also accelerating adoption across homecare settings and among younger, tech-savvy demographics.
AI Integration Enhances Predictive Care and Personalization
Artificial intelligence and advanced analytics are transforming glucose monitoring from reactive to predictive care. AI-enabled systems now detect pattern shifts, forecast glycemic fluctuations, and deliver personalized alerts, reducing hospitalizations and supporting healthier outcomes. As AI models evolve, they increasingly integrate lifestyle data, medication adherence, and sleep patterns, giving patients and clinicians a more holistic view of glucose management.
These innovations not only enhance patient safety but also contribute to lower care costs—strengthening the appeal of smart monitoring systems across public health institutions.
Hospitals Maintain Dominant Role as End Users
Hospitals are set to account for 48.2% of revenue in 2025, making them the largest end-user segment. Intensified diabetes-related hospitalizations and rising surgical volumes have increased the need for dependable continuous monitoring technologies. Hospital procurement teams also benefit from supportive reimbursement structures and prefer clinically validated systems capable of integration with electronic medical records and connected care platforms. This ensures consistency in data, faster intervention rates, and improved patient management across intensive care settings.
Subscribe for Year-Round Insights → Stay ahead with quarterly and annual data updates -
https://www.futuremarketinsights.com/reports/brochure/rep-gb-1183
The Glucose Sensor Market is on track for substantial expansion as global healthcare systems intensify their focus on diabetes management and continuous monitoring. Valued at USD 9.7 billion in 2025, the market is projected to reach USD 22.2 billion by 2035, reflecting a steady 8.5% CAGR. With non-invasive sensors leading product adoption and demand accelerating across North America, Asia-Pacific, and Europe, growth prospects remain firmly positive.
Explore trends before investing – request a sample report today!
https://www.futuremarketinsights.com/reports/sample/rep-gb-1183
Rising Disease Burden and Real-Time Monitoring Fuel Market Expansion
Increasing global diabetes prevalence, paired with the shifting emphasis toward proactive care, is significantly driving market penetration. Hospitals, clinics, and homecare users increasingly recognize the value of continuous, real-time glucose readings in reducing complications, improving glycemic control, and supporting long-term health outcomes. Parallel to this, technological advancements—such as microfluidic sensing, optical technologies, and seamless wearable integrations—are boosting accuracy and consumer acceptance.
As government health bodies reinforce reimbursement policies supporting remote and continuous monitoring, adoption rates continue to climb. This convergence of medical need, policy direction, and emerging technology forms a powerful catalyst for sustained market growth.
Non-Invasive Innovations Unlock a New Era in Monitoring
Non-invasive glucose monitoring is emerging as the most influential trend shaping the industry. This category is projected to capture 57.9% of total market share in 2025, driven by the rising consumer preference for painless, easy-to-use solutions. New-generation sensors utilizing optical and transdermal technologies are entering the mainstream, delivering measurements without finger pricks or implanted components.
The appeal of these devices extends beyond comfort—they offer continuous data streams, reduce infection risks, and increase adherence rates. Wearable platforms launched in 2025, offering rechargeable systems and mobile app connectivity, are also accelerating adoption across homecare settings and among younger, tech-savvy demographics.
AI Integration Enhances Predictive Care and Personalization
Artificial intelligence and advanced analytics are transforming glucose monitoring from reactive to predictive care. AI-enabled systems now detect pattern shifts, forecast glycemic fluctuations, and deliver personalized alerts, reducing hospitalizations and supporting healthier outcomes. As AI models evolve, they increasingly integrate lifestyle data, medication adherence, and sleep patterns, giving patients and clinicians a more holistic view of glucose management.
These innovations not only enhance patient safety but also contribute to lower care costs—strengthening the appeal of smart monitoring systems across public health institutions.
Hospitals Maintain Dominant Role as End Users
Hospitals are set to account for 48.2% of revenue in 2025, making them the largest end-user segment. Intensified diabetes-related hospitalizations and rising surgical volumes have increased the need for dependable continuous monitoring technologies. Hospital procurement teams also benefit from supportive reimbursement structures and prefer clinically validated systems capable of integration with electronic medical records and connected care platforms. This ensures consistency in data, faster intervention rates, and improved patient management across intensive care settings.
Subscribe for Year-Round Insights → Stay ahead with quarterly and annual data updates -
https://www.futuremarketinsights.com/reports/brochure/rep-gb-1183